Nanofabrication of ordered multilayers by alternate adsorption of polyions, nanoparticles and proteins: From planar films to microtemplates.
Yuri M. LVOV
Institute for Micromanufacturing, LaTech, Ruston, LA 71272
A layer-by-layer (LbL) assembly of alternating layers of oppositely charged polyelectrolytes and nanoparticles provides the formation of 5 –500 nm thick films with monolayers of various substances growing in a pre-set sequence on any substrates at a growth step of about 1 nm. This technique was called “molecular beaker epitaxy” meaning by this that with simple instruments (exploiting the materials self-assembly tendency) one can get molecularly organized films similar to the ones obtained with highly sophisticated and expensive molecular beam epitaxy technology for metals and semiconductors. LbL films can coat solid supports, slides, silicon wafers, plastics and fiber optics (2D nanoassembly). Besides, LbL films can be assembled on micro- and nanotemplates, such as 100-500 nm diameter latex, drug microcrystals, biological cells and even viruses, providing by this method for 3D nanoassembly. Polymeric nanoshells also can be prepared with LbL assembly.
1. LbL Self-Assembly Procedure.
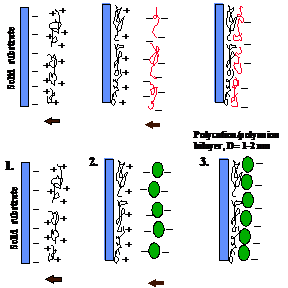
A cleaned substrate of any shape and dimension is immersed into a dilute solution of a cationic polyelectrolyte, for a time optimized for the adsorption of a monolayer (ca 1 nm thick), then is rinsed and dried. The next step is the immersion of the polycation covered substrate into a dilute dispersion of polyanions or negatively charged nanoparticles (or any other nanosize charged species) also for a time optimized for the adsorption of a monolayer, then rinsed and dried. These operations complete the self-assembly of a polyelectrolyte monolayer and monoparticulate layer sandwich unit onto the substrate (Fig. 1). Subsequent sandwich units are self-assembled analogously. Different nanoparticles, enzymes and polyions may be assembled in the pre-planned order in one film.
Forces between nanoparticles and binder layers govern the spontaneous layer-by-layer (LbL) self-assembly of ultrathin films. The forces are primarily electrostatic and covalent in nature, but they can also involve hydrogen bonding, hydrophobic and other types of interactions. The properties of the self-assembled multilayers depend on the choice of the building blocks used, their rational organization and integration along the axis perpendicular to the substrate.
The sequential adsorption of oppositely charged colloids was reported in a seminal paper in 1966 by R. Iler (1). Self-assembly was subsequently “rediscovered” in the nineties and extended to the preparation of multilayers of polycations and phosphonate ions, as well as to the layering of polyelectrolytes (2-5). Self-assembly is now employed for the fabrication of ultrathin films from charged polymers (polyions) and nanoparticles (metallic, semiconducting, magnetic, ferroelectric, insulating), nanoplates, proteins, dyes and other supramolecular species (4-11). That any of these species in any order can be adsorbed layer-by-layer is the greatest advantage of self-assembly. The oppositely charged species are held together by strong ionic bonds and form long-lasting, uniform and stable films which are often impervious to a solvent. Self-assembly is economical and readily amenable to scaling-up for the fabrication of large-area defect-free devices on virtually any kind and shape of surfaces.
The main idea of the method consists of the resaturation of polyion adsorption, resulting in the alternation of the terminal charge after each layer deposition. This idea is general and implies that there is no principle restriction on the choice of polyelectrolytes. It is possible to design composite polymeric films in the range of 5 to 1000 nm, with precision better than 1 nm and a definite knowledge of their compositions. For the successful assembly of nanoparticle or protein multilayers, an alternation with linear or polyion layers is important. Flexible linear polyions penetrate between nanoparticles and act as electrostatic glue. The concept of “electrostatic polyion glue”, which keeps together neighboring arrays of nanoparticles, is central to this approach (10-12).
Standard assembly procedure. As a standard approach to film preparation we use the following steps: 1) Take aqueous solutions of polyion, nanoparticles or protein at a concentration of 0.1 - 1 mg/mL and adjust the pH in such a way that the components are oppositely charged. 2) Take a substrate carrying a surface charge (e.g., plates or polymer films covered by a layer of cationic poly(ethylenimine) which may be readily attached to many surfaces). 3) Carry out alternate immersion of the substrate in the component’s solutions for 10 min with 1 min intermediate water washing. To wash a sample use a solution with pH that keeps the polyions ionized. 4) Dry the sample using a stream of nitrogen (note: drying may disturb the assembly process, and it is not necessary for the procedure).
Polyions predominately used in the assembly are as follows: polycations - poly(ethylenimine) (PEI), poly(dimethyldiallylammonium chloride) (PDDA), poly(allylamine) (PAH), polylysine, chitosan; polyanions - poly(styrenesulfonate) (PSS), poly(vinylsulfate), poly(acrylic acid), heparin, DNA. One can grow polymer nanocomposites with necessary alternation of different material monolayers using hundreds of commercially available polyions.
Fig. 1 Schemes of polycation/polyanion alternate assembly: linear polyanion and polycation and polycation and nanoparticles
 |
Multilayer structure. X-ray reflectivity measurements of polyion films adsorbed from aqueous solutions show patterns with profound intensity oscillations. From the periodicity of these oscillations (Kiessig fringes), one can calculate the film thickness (2-4), and knowing the number of the adsorption steps to get a single polyion layer thickness. The growth steps for a bilayer of 1.1 - 2.0 nm are typical for linear polyanion / polycation assemblies, while a thickness of one layer is often equal to half this value. These values correspond to a polyion cross-section and show that in one cycle of excessive adsorption we have approximately one monolayer coverage on the substrate. The nanoparticle / polyion bilayer thickness is determined by the diameter of the particle. Polyion films are insoluble in water and in many organic solvents and are stable to least 200o C. X-ray reflectivity, atomic-force microscopy and scanning electron microscopy data reveal a film surface roughness of 1 - 2 nm for polymeric films.
The thickness of each individual layer, and thus, also the total thickness of the film can be adjusted precisely by changing the ionic strength of the solution from which the polyions are adsorbed (2-4). Neutron reflectivity analysis of the films composed of alternate layers of deuterated poly(styrenesulfonate) (PSS) and hydrogen containing polyallylamine (PAH) has proved that polyanion / polycation films possess not only uniform thickness, but a multilayer structure as well. The interfaces between layers in polyion films are not sharp and a partial interpenetration (30 % of their thickness) between neighboring polymeric layers will take place (2). A distinct spatial component separation may be reached between the first and the third or fourth neighboring polyion layers. Direct zeta-potential measurements have confirmed a symmetric positive / negative change of the polycation / polyanion multilayer outermost charge with adsorption cycles.
2. Nanoparticle / Polyion Multilayers.
The construction of organic / inorganic nanostructured materials is an important target of modern materials research. An alternate adsorption procedure was used for the following charged nanoparticles: clay and ceramic plates, nanotubules, 10, 20, 45, 75-nm silica spheres, 50, 150, 300-nm latex, 15-nm gold, 30-nm magnetic Fe3O4, 50-nm CeO2, MnO2, 30-nm TiO2 particles (13-19), as well as 34-nm diameter spherical plant viruses (12). The number of particle monolayers in such “sandwiches” are exactly known, and any profile across the film can be constructed with a resolution of 5-10 nm. Our experience shows that using a "soft" polymeric interlayer was important for the composite multilayers formation: flexible linear or branched polyions optimize electrostatic attraction. Nevertheless, after the assembly is completed, organic interlayers can be removed by a 2-hour thermal treatment at 300° C on air (calcination process (20)), which results in direct contacts between the nanoparticles needed for improved electric or magnetic properties. Nanoparticles, such as gold, silver, and fullerenes may be “sandwiched” in multilayers with proteins providing electrically or optically induced electron donor-acceptor properties. Semiconductor nanoparticles, such as CdS, CdSe, were used in the assembly (18-19)
.Silica Multilayers. As an example of the nanoparticle architectures let us analyze a 45-nm silica assembly by alternate adsorption with polycation poly(dimethyldiallylammonium chloride), (PDDA). In situ quartz crystal microbalance (QCM) monitoring of alternate PDDA and SiO2 adsorption gives the following steps:. First, PDDA was adsorbed onto a Ag electrode. The QCM frequency decreased during the first 60 s, after which a slower change was observed as adsorption saturation set in. Then, the resonator was immersed in pure water (black dots) for washing. Next, the film was immersed in a SiO2 dispersion and silica adsorption saturation occurred within several seconds. After subsequent water washing, the film was immersed again in PDDA solution, and so on. Each step was reproducible, and the adsorption process reached 90 % saturation in 10 s for SiO2 and 30 s for PDDA. At every assembly step the component monolayers were formed, as was recorded by scanning electron microscopy (SEM) and ellipsometrical studies (Fig. 2).
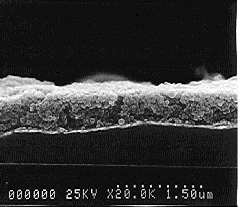

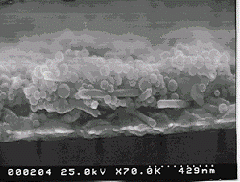
Fig. 2 a. SEM cross-sectional view of {(PDDA + (45-nm SiO2 / PDDA)24 }-film deposited using the 2 s/20 s component adsorption on silver electrode. b. Micropatterning of PDDA/silica multilayers. Strips are of 5 µm width [ref 34].
The average density of SiO2/PDDA multilayers is <r> = 1.43 ± 0.05 g/cm3. SiO2/PDDA film volume composition is: 60 % SiO2 + 10 % polycation + 30 % air-filled pores. These pores are formed by closely packed 45-nm SiO2 and have a typical dimension of 20 nm. The films have controlled pores which could be varied by the selection of the nanoparticle diameter.
We estimated the diffusion limitation for surface coverage A(t) by adsorption from solution of particles with diffusion coefficient D from A(t) = 2/p C √Dt. For t = 2 s, C = 10 mg/cm3 and assuming for 45-nm silica D = 1.1 10-7 cm2/s, A ≈ 3 10-6 g/cm2 and the layer thickness: L = A(t)/<r> ≈ 21 nm. This is reasonably close to the experimental silica monolayer thickness of 24.6 nm. Thus, 2 s corresponds roughly to the diffusion limited time for the SiO2 monolayer adsorption and this time is the fastest nanoparticle monolayer formation rate which we have achieved.
Layered ceramic (plates). Mica-type layered silicates can bear a natural negative charge because of the isomorphous substitution of silicon in octahedral sheets by aluminum or magnesium. The charge is generally balanced by potassium cations that reside in the galleries between layers. The intercalation of organic polymers between sheets of layered ceramics provides an access to novel polymer-ceramic nanocomposites. These nanocomposites exhibit unique physical and mechanical properties attributable to the synergism of the individual components. The build-up of the multilayers in a stepwise manner rather than in the bulk "all-in-once" manner is of special interest. Kleinfeld and Ferguson (13) applied for the first time the electrostatic layer-by-layer adsorption to produce multilayers of anionic synthetic silicate - hectorite and cationic PDDA. We used the polyion assembly to build up multilayers with alternating 1-nm thick montmorillonite sheets and cationic PEI or PDDA (14). The film thickness increase for the montmorillonite adsorption cycle was 1.1 nm and for PEI 2 nm and after 20 cycles the resultant film had a permanent thickness of 63 nm. The construction of ultrathin ceramic films is remarkable, if we take into account that a diameter of a montmorillonite sheet is ca 20 times larger than the film thickness. Two types of defects were visible in the film: the first are due to the adsorption of non-delaminated montmorillonite particles, and the second - to border defects connected with the overlapping of edges of the montmorillonite lamellas.
Tubules. We have described the formation of ordered multilayers from ceramic nanotubules, inorganic spherical particles and enzymes, showing that one can “sandwich” nanoparticles of different shapes and nature in the organized films using linear polyions as electrostatic glue. Halloysite (Al4Si4O10(OH)8 4H2O) is a naturally occurring alumosilicate that exhibits a tubular morphology in the hydrated state. At pH above 4 Halloysite is negatively charged. The tubule morphology of Halloysite exhibits great potential for manipulation in slow-release formulation. The micro-cylinders of the used Halloysite G are of 50 nm in diameter, 300 - 500 nm in length and have 20-nm diameter hollow inner core. Halloysite assembly by sequential adsorption with poly(ethyleneimine) (PEI) or poly(dimethyldiallylammonium chloride) (PDDA) resulted in the formation of ordered multilayers containing from 2 to 20 layers of tubules kept together by polycation interlayers. The tubules in a monolayer are loosely packed; they form a network leaving ca 50 % of empty space. The total thickness of (Halloysite/PEI)14 film was found to be 720 nm and a one-layer thickness is 54±5 nm.
Assembly of tubule / sphere superlattices. In this approach we constructed multilayer by alternating of negatively charged tubules and 45-nm diameter silica spheres, which are kept
Fig. 3 SEM cross-sectional view of the film with alternation of 50-nm diameter Halloysite-G tubules and 45-nm silica spheres. The multilayer architecture was as follows: (PEI / Halloysite)+ (PEI / Halloysite / PEI / silica)3.
Fig. 4 Scanning electron micrographs of {(PEI / PSS)2 + (PEI / glucose oxidase)8 + PEI} film cross-section onto silicon. Conditions: component concentration 3 mg/mL; pH 6.5.
together by polycation interlayers. SEM images confirm the regular alternation of tubules and spheres in the multilayer (Fig. 3).
This is the first example of the manufacture of ordered arrays from differently shaped nanoparticles. The assembly of alcohol dehydrogenase (ADH) and nicotinamide adenine dinucleotide (NAD)-loaded Halloysite multilayers with PEI connecting layers was also achieved. This assembly is targeted for the design of nanocomposites that provide a direct supply of the ADH-cofactor to the enzyme immobilized in polymer films.
3. Protein Multilayers.
Generality of the assembly procedure. Multilayer films which contain ordered layers of protein species were assembled by means of alternate electrostatic adsorption mostly with positively charged PEI, PAH, PDDA, chitosan or with negatively charged PSS, DNA and heparin (12). The pH of the protein solutions was set apart from the isoelectric point so that proteins were sufficiently charged under the experimental conditions. The assembly of 20 different proteins was successfully achieved (including, cytochrome, carbonic anhydrase, myoglobin, hemoglobin, rhodopsin, peroxidase, alcohol dehydrogenase, glucoamylase, glucose oxidase, immunoglobulin, catalase). All the proteins underwent the alternate adsorption with organic polyions for unlimited numbers of cycles (Fig. 4). The mass increment at each step was quite reproducible. Proteins immobilized in multilayers with strong polyions such as PSS, PEI, and PDDA were insoluble in buffer for a pH range between 3 and 10. The assembled proteins are in most cases not denaturated (10-12). Moreover, in some cases the layer-by-layer immobilization with linear or branched polyions enhanced the enzymatic stability.
The enzymatic activity in multilayers increased linearly with the number of layers up to 10-15 protein layers, at which point the film bioactivity became saturated. This saturation was probably due to substrate diffusion limitations into the film, i.e. accessibility to the protein requires a substrate transport through the multilayer (12).
Use of different proteins in alternation with polyions. An elaboration of the assembly technique for a variety of proteins makes it possible to construct multicomponent protein films (superlattices) (12). We have described the formation of two types of superlattices: 1) The alternation of similarly charged proteins at identical pH conditions; both positively charged myoglobin and lysozyme in alternation with polyanion {myoglobin+ / PSS / lysozyme+ / PSS}; negatively charged glucose oxidase (GOx) and glucoamylase (GA) with polycation {GOx- / PDDA / GA- / PDDA}. 2) The combination of negative and positive proteins in the two-block film with the insertion of an additional polyion layer to change the assembly mode: {(lysozyme/PSS)3 + PEI + (GOx/PEI)6. The protein multicomponent films are extremely interesting as novel biologically-active materials. We can arrange given protein layers according to a specific biological activity. Sequential enzymatic reactions and vectorial transfer of electrons and energy become feasible targets by preparing of anisotropic protein layers with the precise control of the distances of active layers.
Sequential catalysis in organized multienzyme films. For the first time, sequential two-step catalysis was demonstrated for glucoamylase (GA) and glucose oxidase (GOx) assembled in the proper order on an ultrafilter through which a substrate (starch) solution was passed (12). Corresponding to the two reaction steps, the outer layer of glucoamylase was followed by a layer of glucose oxidase. Between these two active layers we placed a number of penetrable, but not active, polycation / polyanion layers (PEI/PSS)n (i.e., spacer layers). A 2 % starch solution under 0.5 atm pressure difference moved through the multilayer. The maximal output of the two-step reaction was detected when the upper layer was GA, the bottom layer was GOx, and the distance between them was 10 nm.
4. Nanoparticle Shell Assembly on Microtemplates.
We have discussed polyion film formation on flat solid substrates. The assembly process elaborated for a solid support may be transferred to an assembly on porous carriers (e.g., chromatographic carriers, membranes, porous beads and fibers) or on the surface of charged micro- or nanocores (12, 20-21). The assembly of organized nanoparticle / enzyme / polyion shells on microtemplates is a promising tool for the creation of complex catalytic colloids.
Spherical core. A new approach for building three-dimensional submicron structures using charged latex and self-assembled lipid tubules was recently demonstrated (20-21). In this process, one adds the polycation solution to the negative latex or other core particles. After adsorption saturation is achieved, one has to separate the latex from the polycation solution (usually by 10-min centrifugation at 10,000 g), and then expose the latex to the polyanion solution, and so forth (Fig. 5). In Fig. 6 we present the result of the assembly of 75-nm diameter silica spheres onto 200-nm diameter latex.
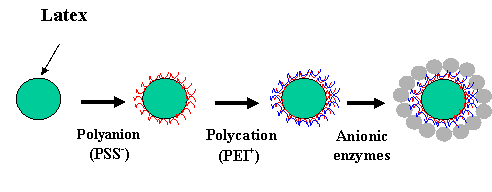
Fig. 5 Scheme of polyion / nanoparticle shell assembly on a spherical microtemplate.

Fig. 6 200-nm diameter latex template and self-assembled 75-nm diameter silica shell on this core.
The assembly was carried out in two steps: first by adsorption of cationic poly(ethyleneimine) we converted the surface charge of negative 200-nm latex to a positive one; and, second, the ordered silica shell was then deposited. Mixing modified and unmodified nanoparticles resulted in their flocculation with the formation of a super-crystal: larger particles in it are separated by a layer of smaller particles.
5. Caps and Helices on Tubules [21].
In another work we used as microtemplates for nanoconstruction 500-nm diameter lipid tubules. First, we used this technique to reveal very small underlying charge distributions on the tubules that were previously not observable. Each new layer of charged polyion multiplies the charge of the layer below, giving a significant (~50-100) amplification process analogous to photography. Final nanoparticle treatment makes this pattern visible in an electron microscope. Second, we used this technique to build up three-dimensional sub micron structures.
Lipid tubules are hollow cylinders made up of bilayer membranes of diacetylenic lipids, with typical diameters of 0.5 mm and lengths of 10-1000 mm. For our purposes, tubules served as templates for the alternate adsorption of charged polymers and nanoparticles. By observing where these charges adsorb on the tubules, we can gain more information about the distribution of charges in tubules, and we can take advantage of tubule helicity to build novel helical structures of nanoparticles. Nanoparticle structures were assembled onto lipid tubules through the sequential adsorption of PSS- and PEI+ and 45-nm silica spheres. For tubules of the zwitterionic 1,2 Di-(10,12-pentacosadiynoyl)-sn-3-phosphatidylcholine) - DC8,11PC, this process leads to the formation of caps on the ends of the tubules, with 50 to 100 silica spheres in each cap (Fig. 7). For tubules of DC8,11PC mixed with 2 % of the charged lipid DC8,9PEOH, the sequential adsorption leads to both end caps and helices of nanoparticles winding around the interior of the tubules (Fig. 8). Further development of this work will be targeted on the nanoconstruction of conductive and magnetic helices.

Fig. 7 TEM image of nanoparticle caps at the ends of tubules of DC8,11PC after four-stage (PEI+ / PSS / PEI+ / 45nm SiO2) treatment.

Fig. 8 Nanoparticle helices inside the tubules of DC8,11PC + 2%DC8,9PEOH after PEI+ / PSS / PEI+ / (45nm SiO2) treatment: TEM image, magnification 5,000; and part of the tubule at magnification 50,000.
6. Bio/Nanoreactor [22-23]
LbL method was applied to form thin organized shells on latex cores. Organized multilayers of nanoparticles (9-, 20-, 45-nm diameter silica or 12-nm magnetite) and glucose oxidase (GOx) were assembled in alternation with oppositely charged polyelectrolytes on 420-nm latex particles. Stepwise growth of the multilayer films on latex was confirmed by microelectrophoresis and transmission electron microscopy (Fig. 9). The shell composition was as following: {(PEI/PSS) + (PEI/magnetite)2 + (PEI/GOx)2}, where PEI - cationic poly(ethyleneimine) and PSS – anionic poly(styrenesulfonate). The inclusion of nanoparticle layers on latex yields a higher surface area, resulting in greater GOx adsorption and thereby increasing the catalytic activity of the bioreactor. The bioactivity was proportional to the core surface area and also to the number of GOx layers in the shells. Also the presence of magnetic nanoparticles allows self-stirring of the nanoreactors with rotating magnetic field and enhances its productivity (Fig. 10).
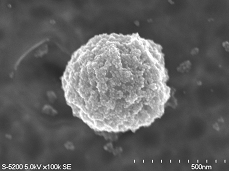
Fig. 9. SEM image of 420-nm latex covered with two monolayers of magnetite particles; less dense GOx layers are not visible.
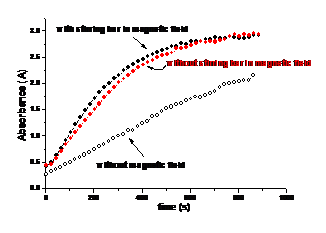
Fig. 10. Time trace of absorbance at 500 nm for the enzymatic activity assay of {(PEI/PSS) + (PEI/12-nm magnetite)2 + (PEI/GOx)2} biocolloids in two different conditions: without magnetic field (circle) and within magnetic field.
In the second approach, after the polycation / polyanion shell was formed, we dissolved the melano formaldehyde latex cores at pH 1 and obtained hollow polymer capsules with wall thickness of 40 nm. These capsules were loaded with enzymes (glucose oxidase or urease). Enhanced biocatalytic activity of such encapsulated enzymes was demonstrated. Enzymes in such biocolloids are protected against high molecular weight denaturation agents and inhibitors while small substrate molecules can readily reach the enzyme.
7. Microencapsulation of biological cells and drug microcrystals [24-25].
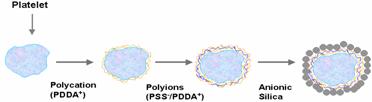
Blood cells, platelets, were coated with 78-nm silica nanoparticles, 45-nm fluorescent nanospheres, or bovine immunoglobulin G (IgG) through layer-by-layer assembly by alternate adsorption with oppositely charged linear polyions (Fig. 11). A sequential deposition on platelet surfaces of cationic PDDA) and anionic PSS was followed by adsorption of nanoparticles or immunoglobulins. Nano-organized shells of platelets were demonstrated by TEM and fluorescence microscope images. Bovine IgG was assembled on platelets, as verified with anti-bovine IgG-FITC labeling. Localized targeting of anti-IgG shelled platelets was also demonstrated. An ability to coat blood cells with nano-organized shells can have applications in cardiovascular research and targeted drug delivery.
Fig. 11. The assembly scheme and SEM image of the shelled platelet.
 |
|||
 |
|||
Furosemide microcrystals were encapsulated with polyions and gelatin to control the release of the drug in aqueous solutions. Charged linear polyions and gelatin were alternatively deposited on 5 mm drug microcrystals through layer-by-layer (LbL) assembly. Sequential layers of PDDA and PSS were followed by adsorption of two to six gelatin / PSS bilayers with corresponding capsule wall thicknesses ranging from 45 to 115 nm. The release of furosemide from the coated microparticles was measured in aqueous solutions of pH 1.4 and 7.4. At both pH values, the release rate of furosemide from the encapsulated particles was reduced by 50-300 times (for capsules coated with 2-6 bilayers) compared to uncoated furosemide (Fig 12). The results provide a method of achieving prolonged drug release through self-assembly of polymeric shells on drug microcrystals.
 Fig. 12 LbL-coated furosemide microcrystal and the drug release from capsules
with wall different wall composition.
Fig. 12 LbL-coated furosemide microcrystal and the drug release from capsules
with wall different wall composition.
Conclusions
 |
We have described the fundamental approach to design organized films that contain different polymer, protein, dye and nanoparticle monolayers in precise locations perpendicular to the surface. The films are amorphous in plane but organized in the third direction with a precision of a few nanometers. Surfaces, porous carriers and fibers of any dimensions, curvatures or complexity may be covered by the film. Microtemplates, such as microspheres, protein nanocrystals and nanotubules, can be shelled with ordered polymer / nanoparticle multilayers. A layer-by-layer assembly is an easy and general process; it does not demand a high purity of components; it can be automated and scaled-up for mass production. This method provides a clear approach to the construction of ordered organic / inorganic nanocomposites.
8. Enzyme Encasing in Polyion Microshells [26-33]
Hollow polyelectrolyte shells assembled through the LbL self-assembly allow precise control of shell size, shell thickness, shell materials, and shell permeability. Lipid bilayers, enzymes and nanoparticles can be included in linear polyion shells.
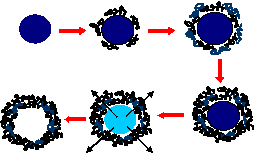
Construction of hollow polyelectrolyte shells involves colloid-templated consecutive polyelectrolyte adsorption followed by decomposition of the templating core. The coating procedure of charged polyions on micro/nano-sized melamine formaldehyde (MF) colloidal particles is illustrated in Fig. 13a. The excess polyelectrolyte in solution can be washed before the next layer is deposited. After the desired polyelectrolyte layers are deposited, the coated particles are exposed to pH1 HCl solution for core decomposition. Hollow shells can be obtained after washing (Fig. 13b).
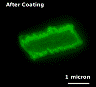
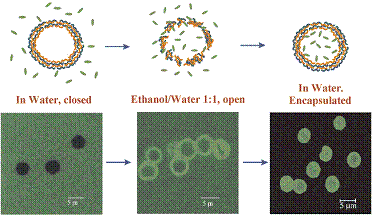
PSS/PAH shells can shift from an “open” state to a “closed” state by changes in environmental conditions such as temperature increase, pH decrease or presence of organic solvents. Currently, two loading mechanisms are used for (PSS/PAH)4-5 shells; first, loading at low pH, and second, loading in mixture of water and alcohol. Shells exposed to a pH 4.5 form holes of up to tens of nanometers in diameter. The opening and closing mechanisms are not fully understood. It is possible that changes of the polyelectrolyte charge upon pH variation induce pore formation or loosen the polyelectrolyte network, thus enabling the polymer to penetrate. Shells incubated in a 1:1 mixture of water and ethanol are sufficiently permeable to allow penetration of 5-nm-diameter urease globules through the shell membrane (Fig. 14). When these same shells are transferred to water, the nanoparticles no longer penetrate. A probable explanation is that the polyion network becomes segregated in the water/ethanol medium, allowing penetration of the urease, while the polyion walls relax to a closed structure when the capsules are returned to pure water.
In a similar approach we encapsulated glucose oxidase, catalase, myoglobin, hemoglobin, and chymotrypsin. Enzyme in the shell have preserved bioactivity and have enhanced stability.
Fig. 13 Scheme of the shell assembly and AFM image of polyion microcapsule with wall composition of (PSS/PAH)4.
 |
Fig. 14. Urease loading in 5-µm diameter (PSS/PAH)4 microcapsule, wall thickness 30 nm.
Literature
1. Iler, R. J. Colloid & Interface Sci., 1966, 21, 569-594 “Multilayers of colloidal particles”
2. Decher, G. Science, 1997, 227, 1232-1237 “Fuzzy nanoassemblies: Toward layered multicomposites”
3. Lvov, Y.; Decher, G; Möhwald, H. Langmuir, 1993, 9, 481-486 “Assembly, structural characterization and thermal behavior of layer-by-layer deposited ultrathin films of polyvinylsulfate and polyallylamine”
4. Lvov, Y.; Haas, H.; Decher, G.; Möhwald, H. J. Phys. Chemistry, 1993, 97, 12835-12841 ”Assembly of polyelectrolyte molecular films onto plasma treated glass”
5. Keller, S.; Kim, H.; Mallouk, T. J. Am. Chem. Soc., 1994, 116, 8817-8818. “Layer-by-layer assembly of intercalation compounds and superlattices on surfaces: Towards molecular "beaker" epitaxy”
6. Cheung, J.; Stockton, W.; Rubner, M. Macromolecules, 1997, 30, 2712-2716 “Molecular-level processing of conjugated polymers. Layer-by-layer manipulation of via electrostatic interactions”
7. Lee, J.; Yoo, D.; Handy, E.; Rubner, M. Appl. Phys. Lett., 1996, 69, 1425-1426 “Thin-film light emitting devices from an electroluminescent ruthenium complex”
8. Stroeve, P.; Vasques, V.; Coelho, M; Rabolt, J. Thin Solid Films 1996, 284, 708-712 "Gas transfer in supported films made by molecular self-assembly of ionic polymers"
9. Hammond, P.; Whitesides, G. Macromolecules, 1995, 28, 7569-7571. “Formation of polymer microstructures by selective deposition of polyion multilayers using patterned monolayers as a template”
10. Ariga, A.; Lvov, Y.; Kunitake, T. J. Am. Chem. Soc., 1997, 119, 2224-2231 "Assembling alternate dye-polyion molecular films by electrostatic layer-by-layer adsorption"
11. Lvov, Y.; J. Schenkman, Rusling, J. J. Am. Chem. Soc., 1998, 120, 4073-4080 “Direct electrochemistry of myoglobin and cytochrome P450 in alternate layer-by-layer films with polyions”
12. "Protein architecture: Interfacial molecular assembly and immobilization biotechnology", Editors: Y. Lvov and H. Mohwald, 2000, Marcel Dekker Publ., NY, p. 1-394.
13. Kleinfeld, E.; Ferguson, G. Science, 1994, 265, 370-373 “Stepwise formation of multilayered nanostructural films from macromolecular precursors”
14. Lvov, Y.; Ariga, K.; Kunitake, T. Langmuir, 1996, 12, 3038-3044 "Formation of ultrathin multilayers and hydrated gel from montmorillonite and linear polycations"
15. Schmitt, J.; Decher, G.; Dressik, W.; Shashidhar, R.; Calvert, J. Adv. Materials, 1997, 9, 61-65 “Metal nanoparticles / polymer superlattice films: Fabrication and control of layer structure”
16. Lvov, Y.; Ariga, K.; Kunitake, T. Langmuir, 1997, 13, 6195-6203 "Alternate assembly of ordered multilayers of SiO2 and other nanoparticles and polyions"
17. Ichinose, I.; H.Tagawa, Lvov, Y.; Kunitake, T. Langmuir, 1998, 14, 187-192 “The formation process of ultrathin films of molybdenum oxide by alternate adsorption of octamolybdate and linear polycations”
18. Cassagneau, T.; Fendler, J.; Adv. Mater, 1998, 10, 877-881 "High density rechargeable Lithium-ion batteries self-assembled from graphite oxide nanoparticles and polyelectrolytes"
19. Cassagneau, T.; Mallouk, T.; Fendler, J. J. Am. Chem. Soc., 1998, 120, 7848-50 “Layer-by-layer assembly of thin film Zener diodes from conducting polymers and CdSe nanoparticles”
20. Caruso, F.; Caruso, R.; Mohwald, H. Science, 1998, 282, 1111-1114 “Fabrication of hollow, spherical silica and shells via self-assembly of nanocomposite multilayers on colloidal templates”.
21. Lvov, Y.; Price, R.; Singh, A.; Selinger, J.; Schnur, J. Langmuir, 2000, 16, in press “Nanoscale patterning on biologically derived microstructures”
22. Y. Lvov, F. Caruso, Analytical Chemistry, 2001, v.73, 4212-4217, “Biocolloids with Ordered Urease Multilayer Shells as Enzymatic Reactors”
23. M. Fang, P. Grant, M. McShane, G. Sukhorukov, V. Golub, Y. Lvov, Langmuir, 2002, v.18, 6338-6344. “Magnetic Bio/Nanoreactor with Multilayer Shells of Glucose Oxidase and Inorganic Nanoparticles”
24. H. Ai, S. Jones, M. de Villiers, Y. Lvov, J. Controlled Release, 2002, v.84, 122-126, “Nanoencapsulation of Furosemide Microcrystals for Controlled Drug Release”
25. H. Ai, M. Fang, S. Jones, Y. Lvov, Biomacromolecules, 2002, v.3, 560-564 “Electrostatic Layer-by-Layer Nano-Assembly on Biological Microtemplates: Platelets”
26. Sukhorukov, G., Donath, E., Lichtenfeld, H., Knippel, E., Knippel, M., Budde, A., and Möhwald, H. Colloids Surfaces A, 1998, 137, 253-266.
27. Moya, S., Donath, E., Sukhorukov, G., Auch, M., Bäumer, H., Lichtenfeld, H., and Möhwald, H. Lipid coating on polyelectrolyte surface modified colloidal particles and polyelectrolyte capsules. Macromolecules, 33, 4538-4544.
28. Sukhorukov, G.B., Antipov, A.A., Voigt, A., Donath, E., and Möhwald, H. pH-controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Commun., 2001, 22, 44-46.
29. G. Sukhorukov, in book: Novel Methods to Study Interfacial Layers”, Ed. D. Moebius and R. Miller, 2001, Elsevier, p.384-415
30. A. Antipov, G. Sukhorukov, S. Leporatti, I. Radchenko, E. Donath, H. Moehwald “Electrolyte multilayer capsule permeability control” Colloids and Surfaces, A, 2002, v. 198, 535-541
31. Sukhorukov, G., Donath, E., Moya, S., Susha, A.S., Voigt, A., Hartmann, J., and Möhwald, H. Microencapsulation by means of step-wise adsorption of polyelectrolytes. J. Microencapsulation, 2000, 17, no 2, 177-185.
32. O. Tiourina, A. Antipov, G. Sukhorukov, Y. Lvov, H. Möhwald, Macromolecular Bioscience, 2001, v.1, 209-214, “Encapsulation of a-Chymotrypsin into the Hollow Polyelectrolyte Microparticles”
33. Y. Lvov, A. Antipov, A. Mamedov, H. Möhwald, G. Sukhorukov, Nano Letters, 2001, v.1, 125-128, “Urease Encapsulation in Nano/organized Microshells”
34. F. Hua, J. Shi, Y. Lvov, T. Cui, Nano Letters, 2002, v.2, 1219-1222, “Patterning of Layer-by-layer Self-assembled Multiple Types of Nanoparticle Thin Films by Lithographic Technique
Patents
·Hong, J-D.; Decher, G. May 4, 1993, US 1991000745572 "One- or multilayered layer elements applied to support and their production;"
·Rubner, M.; Cheung, M. May 21, 1996, US 1993000086548 "Molecular self-assembly of electrically conductive polymers"
·Onda, M; Lvov, Y.; Ariga, K.; Kunitake, T.; February 1, 2000, US 6020175 “Multiple layered functional thin films”