|
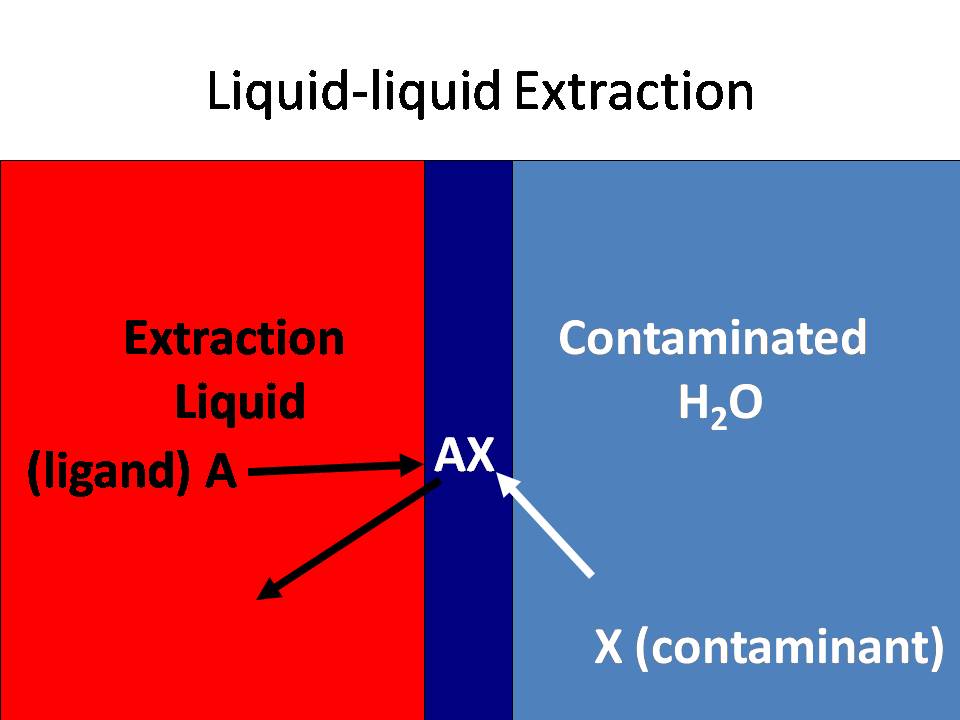
Liquid-liquid extraction is a common technique used to clean up
wastewater by finding a hydrophobic ligand that preferentially
binds to a contaminant, and then brings the contaminant into
another solvent, which is usually an organic liquid, such as
dichloroethane (DCE below) or an ionic liquid.
|
|
|
The uptake of trace atmospheric gases in
aerosols, and the reactions of the gases to form other
products influences general atmospheric composition. The
interface plays a significant role on these processes, and one
of our research goals is to elucidate the importance of the
interface.
|
|
|
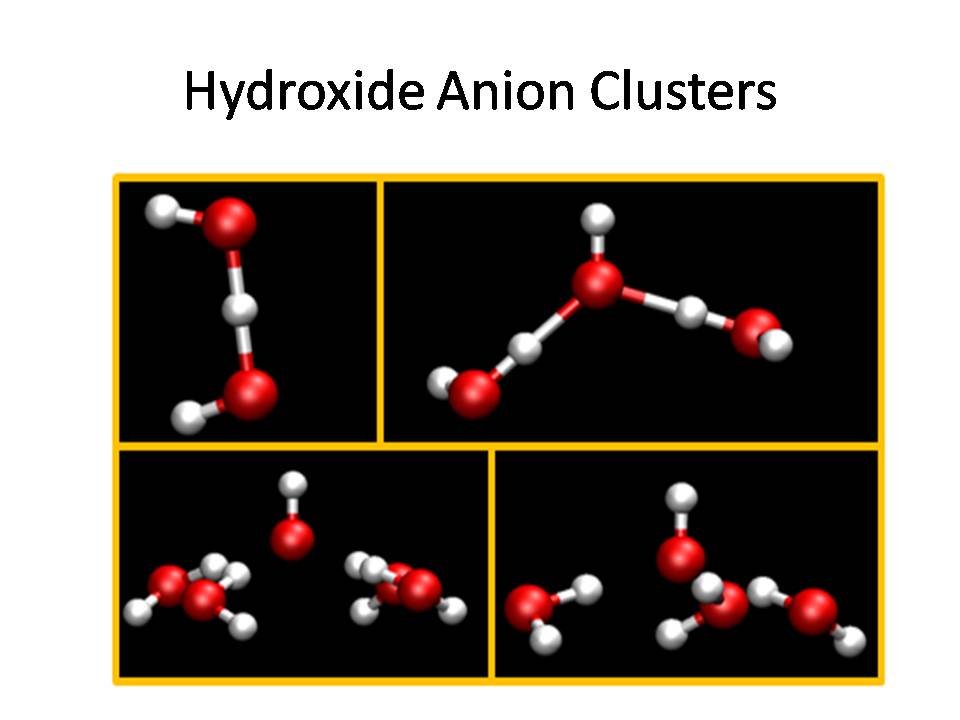
One facet of our research is to understand the
hydroxide anion in solution, which requires a model that
explicitly includes proton sharing between waters and the
hydroxide anion in its implementation.
|
|
|
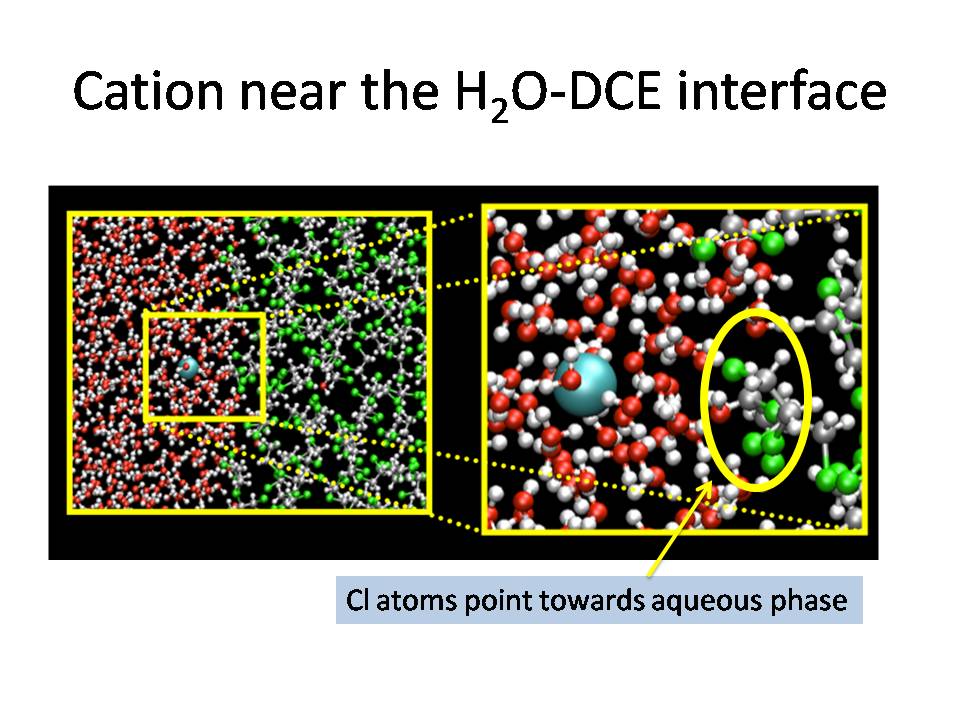
One very common solvent in liquid-liquid
extraction is dichloroethane (DCE), and our research found
that cations have a free energy minimum near the water-DCE
interface.
|
|