LAB 8 - Pool Boiling Heat Transfer
Introduction
Boiling is the term used to describe the phenomena that occurs when evaporation takes place at a solid-liquid interface. The process will occur when the surface temperature Ts exceeds the saturation temperature associated with the liquid pressure Tsat. Heat is transferred to the liquid from the surface according to Newton's law of cooling
(1)
where
= heat flux at the surface
h = heat transfer coefficient
, excess
temperature
Boiling is characterized by the formation of vapor bubbles, which grow and eventually disengage from the surface. The growth of the bubbles and their subsequent dynamics depend upon the excess temperature, the surface characteristics, and on the thermophysical properties of the fluid, the most important of which is the surface tension. The dynamics of the vapor bubble formation influence fluid motion near the surface which affects the heat transfer coefficient.
Boiling may transpire under a variety of conditions. In this experiment, pool boiling will be investigated. This term refers to a condition where the liquid is quiescent and the liquid motion near the surface is due to free convection and to mixing induced by bubble growth and subsequent detachment. Another definition for pool boiling refers simply to conditions in which the heated surface is submerged below a free surface of liquid. Saturated boiling is a condition in which most of the liquid is at a temperature slightly greater than saturation while at the liquid-solid interface there is a significant decrease in liquid temperature. Bubbles that form on the solid surface are driven upward by buoyant forces and eventually are transported to the gas above the free surface. Figure 1 depicts the typical temperature distribution in saturated pool boiling. Subcooled boiling occurs when the liquid temperature is less than the saturation temperature and the bubbles that are formed eventually condense in the liquid.
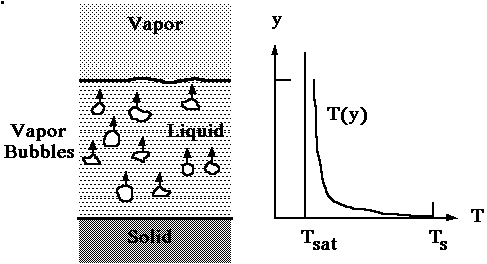
Figure 1. Saturated pool boiling temperature distribution
Saturated pool boiling has received a considerable amount of attention
within the heat transfer community. In this experiment the primary interest
is the boiling curve, which considers the effect of excess temperature
on either the heat transfer coefficient or the heat flux. By studying the
different modes, or regimes, of saturated pool boiling, an appreciation
for the underlying physical mechanisms may be obtained. These regimes are
identified in the boiling curve of Figure 2. The curve shown is for water,
but similar trends are observable in other fluids. Equation 1 indicates
that depends
upon both the heat transfer coefficient h and the excess temperature. The
various boiling modes can be differentiated according to the value of excess
temperature. The different regimes evident on the boiling curve include
film boiling, transition boiling, nucleate boiling, and free convection
boiling. A more complete description of pool boiling and the boiling regimes
can be found in Incropera and DeWitt (1996).
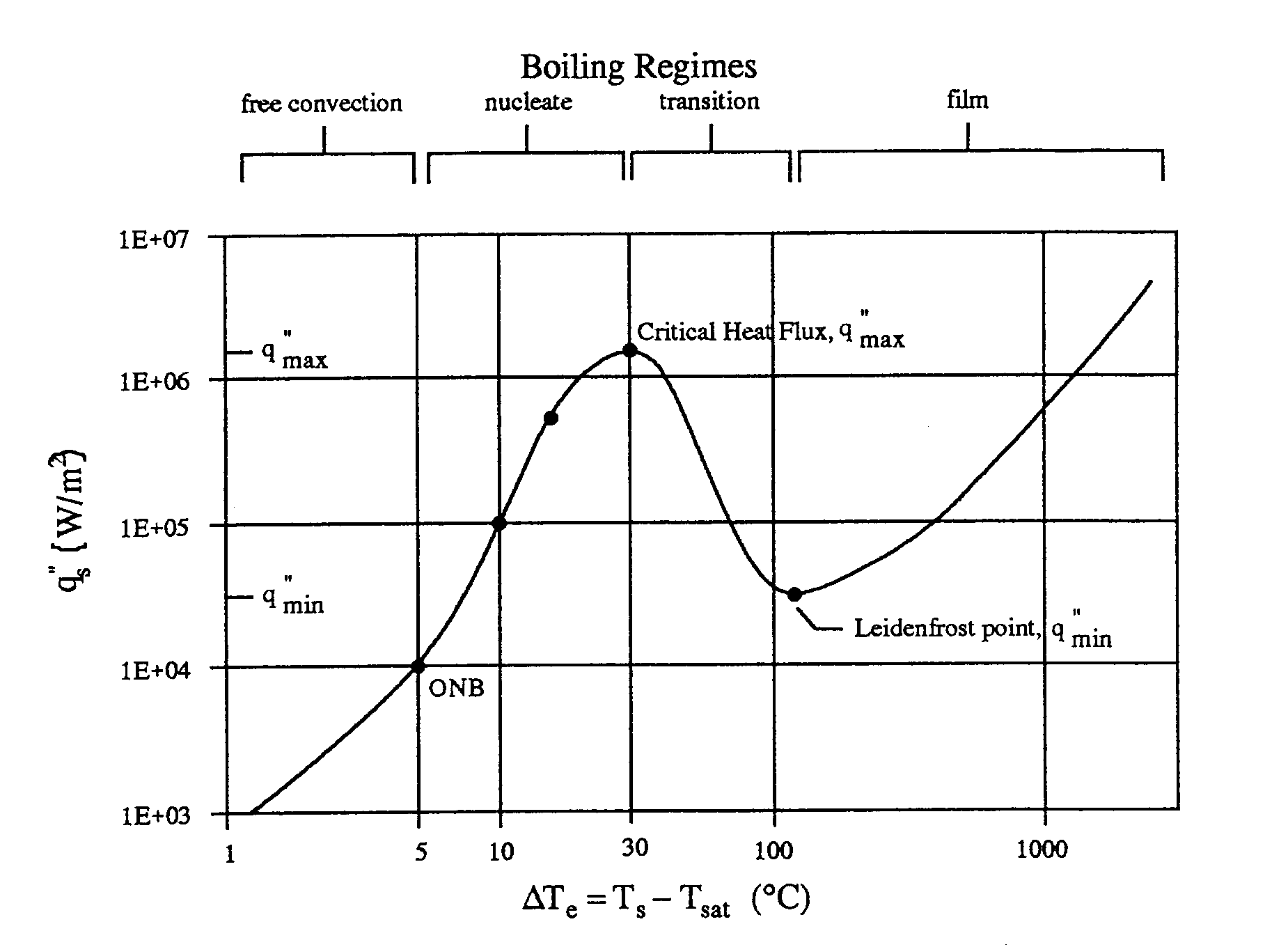
Figure 2. Typical pool boiling curve for water at 1 atmosphere
Objectives
Pool boiling will be investigated in this exercise by immersing an aluminum
cylinder, initially at room temperature, into a container of liquid nitrogen.
The primary objective is to generate a pool boiling curve similar to that
in Incropera and DeWitt (1996), where surface heat flux is given as a function
of the excess temperature .
In addition, the different boiling regimes are to be observed, including
any interesting physical phenomena, and then reported and discussed in
the laboratory report.
Experimental Apparatus
A sufficient supply of liquid nitrogen, such that the aluminum cylinder may be completely immersed, will be contained in a dewar flask. Each student group will be provided with a test cylinder which is approximately 1.5" in diameter and 1.5" in length. A single type-T thermocouple is imbedded in the aluminum cylinders. The cylinders will be suspended from a lab stand at a height that allows the cylinders to be fully immersed in the liquid N2. The thermocouple emf will be read by a HP data acquisition system.
A data acquisition setup has been prepared for the HP system to obtain temperature measurements of the thermocouple every 0.5 seconds. Time and temperature data will be collected for about 5 minutes or until the temperature of the cylinder and liquid nitrogen have reached equilibrium. The data may be exported into a spreadsheet format for analysis. EACH GROUP MUST BRING A FORMATTED DISKETTE TO COLLECT THEIR DATA.
The required thermophysical properties of aluminum are temperature dependent. Data for thermal conductivity and specific heat of aluminum have been fit to a 3rd order polynomial equation in temperature. The general equation has the form
(2)
where T = temperature (°K). Table 1 lists the values of the constants for the two properties of interest.
Table 1. Constants used in Equation 2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The lumped-heat-capacity method may be implemented in order to determine the heat transfer coefficient or surface heat flux as a function of excess temperature. The underlying assumption in this analysis of transient heat conduction is that the entire system is uniform in temperature or, in other words, that the internal resistance is negligible in comparison with the external resistance. Applying the first law of thermodynamics to the body being cooled results in
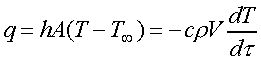
Safety Precautions
Each student must have safety glasses in order to participate in the laboratory exercise. Exercise caution around the liquid N2. Exposing skin to the cold temperature liquid may cause burns.
Procedure
Data Reduction and Points of Interest
References