CHEM 253 Winter 2003
Experiment #2 Dehydration of Cyclohexanol
Background: An alcohol C-OH can be dehydrated (lose water) when heated with a very strong acid (H3PO4). The result is formation of an alkene C=C according to Zaitsev’s rule.
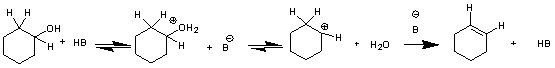
The mechanism is thought to be E1:
Physical Properties:
M.W. density amnt. used mmoles B.P.
Cyclohexanol 100.16 0.963 2.5 mL 160-161oC
Cyclohexene 82.15 0.811 83oC
Phosphoric Acid 98.00 1.70 1.0 mL 158oC (pure)
Calculations:
Caution: Cyclohexanol and cyclohexene are flammable (NO flames allowed) and inhilation, ingestion, or skin absorption may be harmful. Phosphoric Acid can cause serious burns; avoid skin and eye contact.
Glassware and Supplies: 10 mL round-bottom flask, hexagonal magnetic stir bar, Hickman-Hinkle still head, water-cooled condenser, thermometer adapter, thermometer, heating block, stir plate/heater, lab jack, tubing for condenser, stopcock grease, 2 finger-clamps (at least), Pasteur pipet and bulb, centrifuge tube for product
Classroom syringes, needles, automatic pipetters
Procedure: Preweigh: a 10-mL round bottom flask containing the magnetic stirbar and a plastic vial for the product. Place the magnetic stirbar carefully inside the flask! Carefully add 2.5 mL of cyclohexanol and 1.0 mL of phosphoric acid to the flask. Try not to get any liquid on the ground glass joint.
Assemble the distillation apparatus as demonstrated using small amounts of stopcock grease and closing the cap on each joint:
flask with stirbar / Hickman-Hinkle still head / condenser / thermometer and adapter
Position the thermometer bulb just below the collection point on the still head. Be EXTREMELY CAREFUL. Make sure there is a rubber O-ring holding the thermometer into the adapter. Clamp the flask and the condenser to the lab framework such that the lab jack can raise and lower the stirplate/heater to or from the flask. Attach rubber hoses to the condenser and remember to make the water “run uphill” at a gentle trickle. The inlet hose must be attached to a “regular” faucet head.
After placing a heating block on the stirplate/heater, raise the lab jack such that the flask fits into the appropriate well. It needs to be snug but not forcing extra pressure on your glassware. Start the stirrer and begin heating the reactants (start ~2). Double check all connections, and the water flow through the condenser. Control the heating rate so that the product mixture collects slowly in the well of the Hickman-Hinkle still. Record the still-head temperature after distillation begins and observe it at intervals during the reaction. Using a Pasteur pipet, transfer distillate frequently to the centrifuge tube. As the alkene volume approaches 1 mL, monitor the still-head temperature continually. Remove the apparatus from the heat source (lower the lab jack) when you observe a marked temperature drop at the still head, which may be accompanied by foaming and dense white fumes in the reaction flask.
Wash the distillate with 1.5 mL of 5% aqueous sodium bicarbonate (NaHCO3) and carefully remove the aqueous layer with a Pasteur pipet. Dry the alkene with anhydrous calcium chloride for several minutes. Decant (or pipet) the dry alkene into the preweighed vial and weigh the results in order to calculate the percent yield of the reaction. Also weigh the reaction flask after it has cooled to touch.
You may dispose of the aqueous waste in the sink. Please put the reaction waste in the “Waste Organic” bottle. Remember to remove your stirbar and wash it carefully (don’t let it fall in the drain!!!) Turn in your cyclohexene product in the weighed vial.
Carefully disassemble your glassware, wash it with soap and water, and place it back in the kit.
Questions: What was the purpose of the sodium bicarbonate?
Why would you want to “dry” your product?
Show your percent yield calculations for the product.
Can you think of reasons why the yield wasn’t higher?
OR Can you think of reasons why you calculated over 100% yield?