BIEN 435 Laboratory
Simulation of Cardiac
Output Measurement
Null Hypothesis
This
laboratory will be designed to simulate the measurement of cardiac output by
the Fick principle. See the accompanying
description of the Fick principle. The
null hypotheses you would like to test are:
- The measured value of cardiac
output does not depend on the time of injection of the test fluid.
- The measured value of cardiac
output does not depend on the total amount of fluid injected.
In
addition, you will compare the signal that you obtain in the “aorta” to the
derived relationship of concentration (temperature) as a function of time.
Theory
There are
two parts to the theoretical development.
The first is the determination of concentration at the detector as a
function of time. This has been provided
in the handout on the thermal dilution method (Download
MS Word File). The second part
is the determination of flow rate from the measured concentration.
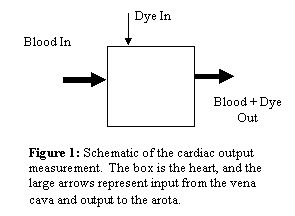
Consider
the situation in Figure 1. Blood is
injected into a mixing chamber along with a dye. The concentration is measured at the outlet
(Blood + Dye Out). Conservation of mass
says that the total mass in must be the total mass out. Total mass in is the concentration in the
syringe multiplied by the total volume injected (cin V). Total mass out is the integral of
concentration times flow rate over time.
Therefore:
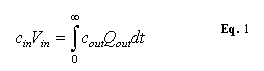
Assume that
the flow rate out is constant with time.
This is a reasonable assumption as long as the amount of fluid injected
is small. Then ![]() can be taken out of the
integral and the equation can be solved for
can be taken out of the
integral and the equation can be solved for ![]() to yield.
to yield.
![]()
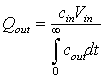
Here, ![]() will be known because
you know the number of drops per unit volume in the injection syringe.
will be known because
you know the number of drops per unit volume in the injection syringe. ![]() is just the amount of
fluid you inject. The integral in the
bottom must be obtained from the trace that you will get on the oscilloscope
(the output of your concentration detection device). You must find a reasonable way of integrating
this trace. One way is to pick values
off of the oscilloscope, fit these to the theoretical curve of concentration as
a function of time, and then integrate the theoretical curve. Another simple method is to trace the curve
on a piece of semitransparent paper, cut out the area under the curve, and
weigh the cut out. You can also simply
do a Simpson’s rule type of integration, again, from the numbers you pick off
the oscilloscope. Whatever method you
pick, you must use your calibration curve for concentration to determine
concentration from the voltage values and then use this to determine
is just the amount of
fluid you inject. The integral in the
bottom must be obtained from the trace that you will get on the oscilloscope
(the output of your concentration detection device). You must find a reasonable way of integrating
this trace. One way is to pick values
off of the oscilloscope, fit these to the theoretical curve of concentration as
a function of time, and then integrate the theoretical curve. Another simple method is to trace the curve
on a piece of semitransparent paper, cut out the area under the curve, and
weigh the cut out. You can also simply
do a Simpson’s rule type of integration, again, from the numbers you pick off
the oscilloscope. Whatever method you
pick, you must use your calibration curve for concentration to determine
concentration from the voltage values and then use this to determine ![]() .
.
Experimental Setup
In this
experiment you will assemble your own experimental system. Schematically, the system will look like the
diagram below: However, it will be up to
you to put this system together. Some of
the pieces are simple, given what you have done already. Certainly, the two reservoirs and the tubing
are already available. You will need to
add something to make sure that the injected dye (or hot/cold water) is mixed
thoroughly with the flowing water. The
concentration detection system can be easily made from a laser, a
photoresistor, a voltage supply and a second resistor. You cannot use the digital mutimeter to take
readings as a function of time because the concentration will change too
quickly to capture. Thus, you will need
to use an oscilloscope.
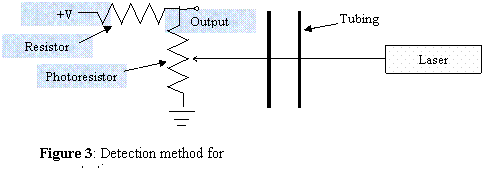
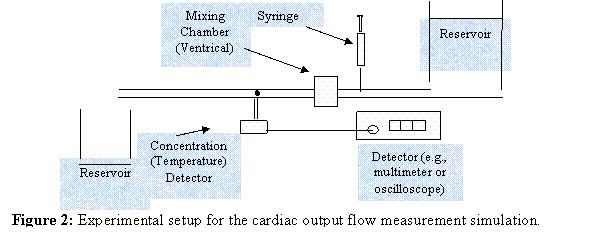 Figure 3 shows a simple
concentration detector circuit. The
photoresistor changes its resistance according to the amount of light incident
on its sensor. As the dye passes in
front of the laser, it attenuates the light, causing less light to impinge on
the photoresistor. The resistor and the
photoresistor form a simple voltage divider so that the output is:
Figure 3 shows a simple
concentration detector circuit. The
photoresistor changes its resistance according to the amount of light incident
on its sensor. As the dye passes in
front of the laser, it attenuates the light, causing less light to impinge on
the photoresistor. The resistor and the
photoresistor form a simple voltage divider so that the output is:
![]()
where ![]() is the resistance of
the photoresistor,
is the resistance of
the photoresistor, ![]() is the resistance of
the resistor, and
is the resistance of
the resistor, and ![]() is the dc voltage
supplied to the circuit. Increased light
casues decreased photoresistor voltage, causing reduced output voltage. Since an increased concentration will
decrease the amount of light, output voltage increases with concentration.
is the dc voltage
supplied to the circuit. Increased light
casues decreased photoresistor voltage, causing reduced output voltage. Since an increased concentration will
decrease the amount of light, output voltage increases with concentration.
Transducer Calibration
You will
need to calibrate your detector. This
should be done in a manner similar to the way you calibrated the pressure
transducer in the previous experiment.
Should the output be linear, based on the circuit design? I is clear from Eq. 3 that the circuit itself
is inherently nonlinear with respect to ![]() , but if
, but if ![]() this nonlinearity can
be minimized. Nonetheless, the
resistance may still be nonlinear with respect to the amount of light impinging
on the photoresistor.
this nonlinearity can
be minimized. Nonetheless, the
resistance may still be nonlinear with respect to the amount of light impinging
on the photoresistor.
To obtain
the calibration, perform a linear least squares fit of concentration as a
function of voltage out. This will
enable you to translate all of the voltage readings directly to concentration.
Remember to
measure the true diameter of the tubing used in this experiment.
Flow Rate
As in the
pressure drop experiment, determine the true flow rate. Use a graduated cylinder to set the flow rate
to approximately this value. Measure the
flow rate as precisely as possible.
Experimental Procedure
To test the
two null hypotheses, you will need to measure concentration as a function of
time for three cases: 1) Standard amount
of injectate over a standard amount of time.
2) Twice the amount of injectate in the same amount of time. 3) Standard amount of injectate over twice
the time. You will need at least 5
repetitions for each case to perform your t-test. You will need to use the storage and single
sweep trigger features of your oscilloscope.
Data analysis
1. Determine the calibration of your
sensor (concentration as a function of voltage).
2. For each data set value of (Volume
Collected, Time of Collection), translate to flow rate (Q)
3. Determine a reasonable way to get
flow rate from the concentration measurements.
This will most likely involve integrating the signal on the oscilloscope
in some way.
4. Plot concentration as a function of
time.
5. Plot the theoretical curve, based on
the experimental values used.
6. Discuss reasons for any differences
(theory vs experiment).
7. Perform Student’s T-tests to test
the two hypotheses. Provide a p-value in
both cases. Are the results significant?